Abstract
Introduction: Patients (pts) with multiple myeloma (MM) who experience early clinical relapse, defined as disease progression ≤12 mo after autologous stem cell transplantation (ASCT) or start of initial treatment (tx), have median overall survival rates <2 y despite tx with novel agents such as proteasome inhibitors (PI), immunomodulatory drugs (IMiDs), and monoclonal antibodies. Ciltacabtagene autoleucel (cilta-cel) is a chimeric antigen receptor (CAR) T-cell therapy with 2 BCMA-targeting single-domain antibodies. In the phase 1b/2 CARTITUDE-1 study, cilta-cel demonstrated deep and durable responses in heavily pretreated pts with relapsed/refractory MM (Berdeja, Lancet , 2021). The phase 2, multicohort CARTITUDE-2 study (NCT4133636) is evaluating the safety and efficacy of cilta-cel in pts with MM in various disease settings. Here, we present the first results from cohort B of CARTITUDE-2, which enrolled pts following early relapse after initial therapy that included a PI and IMiD.
Methods: Eligible pts had MM, received 1 prior line of therapy (PI and IMiD required), had disease progression per IMWG criteria (either ≤12 mo after ASCT or ≤12 mo after start of anti-myeloma therapy for pts who did not undergo ASCT), and were tx-naïve to CAR-T or anti-BCMA therapies. A single cilta-cel infusion (target dose 0.75×10 6 CAR+ viable T cells/kg) was given 5-7 d after start of lymphodepletion (300 mg/m 2 cyclophosphamide and 30 mg/m 2 fludarabine daily for 3 d). The primary objective was minimal residual disease (MRD) negativity at 10 -5, as assessed by next generation sequencing. Adverse events (AEs) were graded using CTCAE v5.0 (cytokine release syndrome [CRS]) and immune effector cell associated neurotoxicity syndrome (ICANS) by ASTCT criteria. To minimize risk of movement and neurocognitive tx-emergent AEs (TEAEs), pt management strategy was implemented, consisting of enhanced bridging therapy to reduce baseline tumor burden, early aggressive tx of CRS and ICANS, and handwriting assessment tools for early detection of neurotoxicity symptoms.
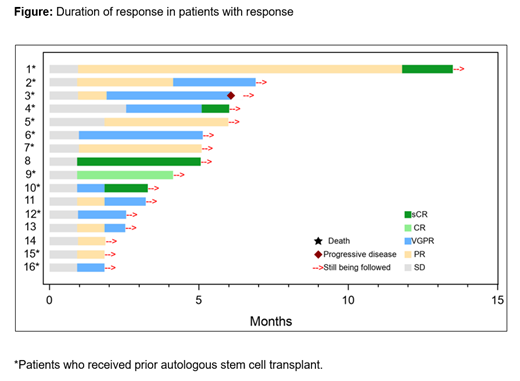
Results: As of the April 15, 2021, data cutoff, 18 pts (median age 57.0 y [range 44-67]; 78% male) received cilta-cel, and 2 pts died before cilta-cel infusion (1 each due to progressive disease and worsening general status). Median follow-up was 4.7 mo (range 0.6-13.5); median time from diagnosis to enrollment was 1.1 y (range 0.5-1.9). Two (11.1%) pts had high cytogenetic risk and 5 (27.8%) had bone marrow plasma cells >30%; 14 (77.8%) pts received prior ASCT, and 15 (83.3%) were refractory to their prior therapy. Overall response rate was 88.9% (95% CI: 65.3-98.6), 27.8% of pts (95% CI: 9.7-53.5) achieved ≥complete response (CR), and 66.7% (95% CI: 41.0-86.7) achieved ≥very good partial response (VGPR). Median time to first response was 0.9 mo (range 0.9-2.6), median time to best response was 1.4 mo (range 0.9-11.8), and median time to ≥CR was 1.8 mo (range 0.9-11.6). Of the 13 pts with ≥3 mo follow up, 5 (38%) achieved ≥CR. Of pts who were MRD-evaluable (n=9), all were MRD 10 -5 negative (100% [95% CI: 66.4-100]). At data cutoff, all but 1 pt remained in clinical response (Figure). Hematologic TEAEs in ≥20% of pts were neutropenia (88.9%), thrombocytopenia (61.1%), anemia (50.0%), leukopenia (27.8%), and lymphopenia (22.2%). CRS occurred in 15 (83.3%) pts (1 gr 4); median time to onset was 8 d (range 5-11), consistent with the heavily pretreated pts in the CARTITUDE-1 study. Median duration of CRS was 4 d (range 1-7). ICANS (gr 1) occurred in 1 pt. One pt experienced movement and neurocognitive TEAEs (gr 3) on Day 38 post cilta-cel infusion. This pt had high disease burden at baseline, progressive disease despite triplet bridging therapy, and gr 4 CRS which have been identified as risk factors for movement and neurocognitive TEAEs. The pt was treated with immune-directed measures and was reported to be stable with some improvements at data cutoff and achieved VGPR. No study deaths occurred post cilta-cel infusion.
Conclusions: A single cilta-cel infusion led to early and deep responses in pts who experienced early clinical relapse/tx failure to initial therapy, with a manageable safety profile. MRD negativity was achieved early. Responses continue to deepen, and follow-up is ongoing, including MRD assessment. These findings support the continued exploration of cilta-cel in earlier lines of treatment and incorporation into potentially curative front-line regimens.
Van de Donk: BMS/Celgene: Consultancy, Honoraria; Janssen: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Cellectis: Research Funding; Takeda: Consultancy; Roche: Consultancy; Novartis /bayer/servier: Consultancy. Delforge: Amgen, Celgene, Janssen, Sanofi: Honoraria, Research Funding. Cohen: Takeda: Consultancy; Janssen: Consultancy; BMS/Celgene: Consultancy; Novartis: Research Funding; AstraZeneca: Consultancy; GlaxoSmithKline: Consultancy, Research Funding; Genentech/Roche: Consultancy; Oncopeptides: Consultancy. Cohen: GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm: Research Funding; Neopharm / Promedico: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau. Hillengass: Curio Science: Speakers Bureau; Beijing Medical Award Foundation: Speakers Bureau; Beijing Life Oasis Public Service Center: Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Oncotracker: Membership on an entity's Board of Directors or advisory committees; Skyline: Membership on an entity's Board of Directors or advisory committees; Adaptive: Membership on an entity's Board of Directors or advisory committees; Axxess Network: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees. Kerre: BMS: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Elyad: Membership on an entity's Board of Directors or advisory committees. Schecter: Janssen: Current Employment, Current holder of stock options in a privately-held company. De Braganca: Janssen: Current Employment. Varsos: Janssen: Current Employment. Vogel: Janssen Global Services, LLC: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months. Zudaire: Janssen: Current Employment. Corsale: Janssen: Current Employment. Akram: Legend Biotech USA: Current Employment. Geng: Legend Biotech USA: Current Employment. Nesheiwat: Legend Biotech USA: Current Employment. Pacaud: Legend Biotech: Current Employment. Sonneveld: Karyopharm: Consultancy, Honoraria, Research Funding; SkylineDx: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding. Zweegman: Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding.
At the time of abstract submission, cilta-cel is being investigated for the treatment of multiple myeloma but is not yet approved.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal